Understanding X chromosome inactivation is pivotal in the realm of genetics, particularly when exploring the intricacies of genetic disorders linked to this chromosome. This unique phenomenon allows females, with their two X chromosomes, to silence one copy to maintain a balanced gene dosage with males, whose single X chromosome does not require such regulation. Recent findings coming from the Jeannie Lee lab shed light on the mechanisms of chromosomal silencing, paving the way for innovative treatments for conditions like Fragile X Syndrome and Rett Syndrome. By targeting the gelatinous substance that facilitates this inactivation, researchers are uncovering potential therapies that could revolutionize how we approach these genetic disorders. The ongoing Rett Syndrome research and Fragile X Syndrome treatment initiatives are not only addressing symptoms but also aiming to unlock the therapeutic potential of previously silenced genes.
The study of X chromosome inactivation, also known as Lyonization, is central to our understanding of gender-based genetic expression differences. Female mammals typically exhibit two X chromosomes, leading to a complex regulation process that silences one X to prevent an overload of gene expression compared to males. The latest research from the Jeannie Lee laboratory emphasizes the critical role of this chromosomal silencing in potentially unlocking treatments for genetic disorders, particularly those associated with the X chromosome. This endeavor, focused on mechanisms of gene regulation, ties into ongoing efforts to develop effective therapies for disorders like Fragile X and Rett Syndrome. By harnessing the power of genetic therapies and chromosomal regulation, scientists are paving the way for new approaches in treating these conditions.
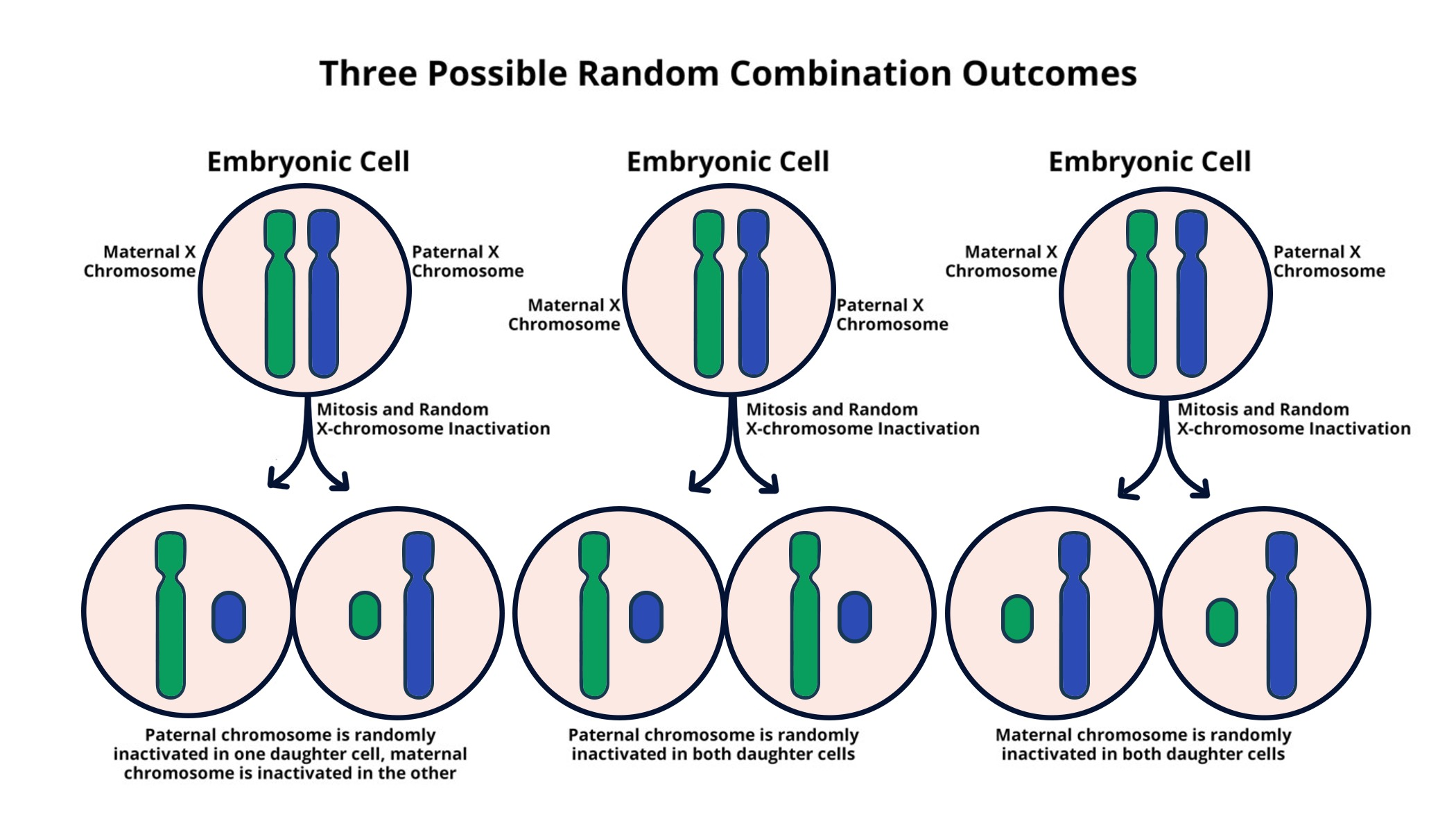
The Mechanism of X Chromosome Inactivation
X chromosome inactivation is a crucial biological process that balances gene dosage between males and females. In females, where two X chromosomes are present, one of the two copies undergoes a silencing mechanism. This inactivation is facilitated by a non-coding RNA called Xist, which plays a pivotal role in modifying the chromosomal landscape. The process involves the formation of a gel-like substance that segregates the chromosomal material, ensuring that the genes from one X chromosome remain inactive while the other is active. This mechanism is not only pivotal for understanding genetic expression but also holds considerable significance in the context of X-linked genetic disorders.
Recent studies by Jeannie Lee’s lab illustrate how the interplay between Xist and the gel-like substance aids in the efficient silencing of the inactivated X chromosome. By altering the physical properties of this gelatinous matrix, Xist enables the incorporation of additional molecules that further bind to the inactive X chromosome, facilitating its silencing. Understanding this intricate interplay is not only essential to decipher basic biological processes but also offers pathways to potential therapeutic interventions for conditions like Fragile X Syndrome and Rett Syndrome, which are directly linked to mutations on the X chromosome.
Advancements in Fragile X Syndrome Treatment
Fragile X Syndrome, one of the most common inherited causes of intellectual disability, is characterized by specific mutations on the FMR1 gene located on the X chromosome. Recent breakthroughs in the field demonstrate promising therapeutic avenues for individuals affected by this disorder. Jeannie Lee and her team are at the forefront of research aimed at correcting the silencing of mutated genes. By leveraging the findings on X chromosome inactivation, they explore novel methods to ‘unsilence’ the FMR1 gene, potentially restoring its function, which would lead to significant improvements in cognitive abilities and overall quality of life for patients.
The ongoing research not only focuses on direct treatment methodologies but also encompasses understanding the broader implications of chromosomal silencing in Fragile X Syndrome. By creating conditions that allow the reactivation of the silenced FMR1 gene, scientists hope to offer relief to those affected. Moreover, this research may pave the way for similar therapeutic strategies addressing other X-linked disorders, thereby expanding the scope of genetic disorders therapy. As studies progress towards clinical trials, the hope builds that these advancements will yield new treatment paradigms in the near future.
Exploring Rett Syndrome: A Breakthrough in Research
Rett Syndrome is a neurodevelopmental disorder predominantly affecting females and is often caused by mutations in the MECP2 gene on the X chromosome. Given its profound impact on neurological development, understanding the mechanics behind Rett Syndrome has been a primary focus in genetic research. Jeannie Lee’s lab is pioneering these efforts by examining how the processes of X chromosome inactivation interplay with gene mutations associated with this disorder. Their work aims to identify potential pathways for therapeutic intervention, which could positively impact the lives of those diagnosed with the syndrome.
The potential of reversing the effects of Rett Syndrome through targeted therapies is immensely promising. By applying the foundational principles learned from studies on Xist, researchers can establish innovative strategies to restore normal gene function. Just as in their investigations related to Fragile X Syndrome, the understanding of chromosomal silencing presents a unique opportunity to engineer treatments for Rett Syndrome. Continuous investment in this area can illuminate new therapeutic practices, offering hope to families impacted by neurodevelopmental disorders.
The Role of Chromosomal Silencing in Genetic Disorders
Chromosomal silencing, particularly through the mechanism of X chromosome inactivation, plays a critical role in regulating gene expression in cases of genetic disorders. This process ensures that although females possess two copies of the X chromosome, only one remains active in each cell. Understanding how cells control this process provides insight into various genetic disorders, including Fragile X Syndrome and Rett Syndrome. Researchers like Jeannie Lee emphasize the importance of this silencing mechanism, as it not only maintains genomic balance but also presents targets for therapeutic approaches.
As researchers uncover the complexities of chromosomal silencing, it becomes increasingly clear that these insights may lead to effective genetic disorders therapy. The emerging strategies to modulate gene expression could pave the way for groundbreaking treatments that specifically address the underlying causes of these disorders, rather than just managing symptoms. With continued research and development, there is potential not only to improve outcomes for patients but also to expand the understanding of how chromosomal mechanisms impact broader aspects of human health.
Jeannie Lee’s Lab: Pioneering Genetic Research
The lab of Jeannie T. Lee at Harvard Medical School stands at the forefront of genetic research, particularly in the study of X chromosome inactivation. For over two decades, Lee’s team has diligently pursued the fundamental question of how X-inactivation occurs at the cellular level. Their groundbreaking findings have now reached a phase where the insight gained can translate into therapeutic applications, particularly concerning conditions linked to X-linked mutations such as Fragile X Syndrome and Rett Syndrome. The dedication to deciphering these mechanisms represents a significant stride in molecular biology and genetics.
Lee’s work exemplifies the critical intersection of basic and applied research, showcasing how understanding the nuances of chromosomal behavior can lead to direct clinical applications. By theorizing that successful intervention could unbind mutated genes from their inactivated state, her lab opens up new discussions surrounding therapeutic strategies that could eventually be utilized in broader genetics research. As they work towards clinical trials, the potential impact of their findings on the treatment landscape for genetic disorders becomes increasingly palpable, offering a beacon of hope for affected families.
The Future of Genetic Disorder Therapies
The future of genetic disorder therapies seems promising, especially with recent findings related to X chromosome inactivation and chromosomal silencing. As research progresses, the innovations stemming from Jeannie Lee’s lab are expected to transform the therapeutic landscape for patients suffering from X-linked diseases. The potential to unsilence genes that have remained dormant due to inactivation not only represents a groundbreaking approach but also redefines the boundaries of what genetic treatment can achieve. Such advancements encourage researchers to think innovatively about genetic therapy beyond traditional paradigms.
Continued exploration of chromosomal mechanisms will likely lead to further understanding of how to manipulate gene expression, providing a pathway toward effective treatments for diseases that currently have no cure. The collaborative efforts among researchers, clinicians, and funding bodies will be essential to realize the potential of these therapies, ensuring that they are safe and effective for patients. Ultimately, the trajectory of research suggests an exciting era where genetic disorders may cease to be life-long challenges and instead become manageable or even curable conditions.
The Importance of NIH Support in Genetic Research
The support from the National Institutes of Health (NIH) has played a pivotal role in advancing research on genetic disorders, including those associated with X chromosome inactivation. For over 25 years, Jeannie Lee’s lab has benefited from NIH funding, allowing for in-depth exploration into complex biological questions that underpin genetic disorders. This financial backing has empowered researchers to uncover fundamental insights about gene function and expression, laying the groundwork for future therapeutic approaches. Without such funding, many of the breakthroughs that have emerged might not have been possible.
The sustained investment in genetic research underscores the importance of public funding in catalyzing scientific progress. As researchers explore the intricacies of chromosomal behaviors and their implications for diseases like Fragile X Syndrome and Rett Syndrome, continued support from agencies like the NIH becomes essential in bridging the gap between basic science and clinical application. The future of genetic disorders therapy depends on this ongoing commitment to research, highlighting the critical role that support systems play in translating discoveries into benefits for patients and their families.
Potential Side Effects of Therapeutic Approaches
While the therapeutic potential unlocked by studies on X chromosome inactivation is exceedingly promising, it raises questions about possible side effects. As Jeannie Lee’s research progresses towards clinical trials, understanding how unsilencing a mutated gene impacts the overall genomic environment becomes paramount. One of the encouraging findings so far is that efforts to reactivate mutated genes do not seem to adversely affect healthy gene functions. This suggests that targeted therapies might offer a mechanism for correcting deficits in gene function without disrupting normal cellular operations.
However, the complexity of gene interactions means that thorough investigations will be necessary to map out any unforeseen consequences that may arise from such interventions. Research teams must pay close attention to patient responses to these innovative therapies, ensuring a careful evaluation of potential side effects. By addressing these considerations early on, researchers can enhance the safety profile of new treatments while refining approaches to maximize their effectiveness concerning conditions like Fragile X Syndrome and Rett Syndrome.
The Broader Implications of X Chromosome Research
The research surrounding X chromosome inactivation has broader implications beyond Fragile X and Rett syndromes. The mechanisms of chromosomal silencing shed light on the intricate regulation of gene expression, with potential applications in a wide array of genetic disorders. As scientists like Jeannie Lee unveil the mysteries of how genes are activated and silenced, it opens doors for innovative approaches to treating other inherited conditions. These advances may also enhance our understanding of diverse biological processes, contributing to overall progress in molecular genetics.
Moreover, leveraging the principles of X chromosome research may lead to influential strategies in gene therapy, enabling interventions at a fundamental level. This can potentially alter the trajectory of treatment for genetic disorders, fostering a new vision where previously untreatable conditions may be managed effectively. As these research initiatives continue to evolve, the cascading effects on healthcare and patient outcomes could signal a transformative shift in how we approach genetic disorders therapy in the future.
Frequently Asked Questions
What is X chromosome inactivation and why is it important for genetic disorders therapy?
X chromosome inactivation is a biological process that occurs in females, where one of the two X chromosomes is randomly silenced to ensure that gene dosage is maintained between males (with one X chromosome) and females (with two). This process is crucial for genetic disorders therapy as many conditions, such as Fragile X syndrome and Rett syndrome, are linked to mutations on the X chromosome. Understanding this mechanism can lead to innovative treatments that could activate silenced genes, potentially alleviating the effects of these disorders.
How does Jeannie Lee’s lab contribute to the understanding of X chromosome inactivation?
Jeannie Lee’s lab at Harvard Medical School has been instrumental in researching X chromosome inactivation. Their studies have revealed that chromosomal silencing is influenced by a gelatinous substance surrounding the chromosomes, likened to Jell-O. By studying the mechanisms involved, the Lee lab has identified potential therapeutic approaches for diseases like Fragile X syndrome and Rett syndrome that could restore the function of inactivated X-linked genes.
What role does Xist RNA play in the process of X chromosome inactivation?
Xist RNA is a crucial molecule in the process of X chromosome inactivation. It is produced by a gene on the X chromosome and binds to the chromosome, changing the properties of the surrounding chromosomal material. This leads to chromosomal silencing by preventing the expression of genes on that X chromosome. The manipulation of Xist RNA is now being explored by researchers, including those in Jeannie Lee’s lab, as a potential method for therapies targeting genetic disorders linked to X chromosome mutations.
Can X chromosome inactivation be reversed to treat Fragile X syndrome?
Yes, recent research suggests that it may be possible to reverse X chromosome inactivation to treat conditions such as Fragile X syndrome. Researchers like those in Jeannie Lee’s lab are developing methods to ‘unsilence’ inactivated X chromosomes, allowing access to healthy gene copies that were previously unavailable due to chromosomal silencing. This approach holds promise for effectively treating genetic abnormalities arising from X-linked mutations.
What are the implications of X chromosome silencing for Rett syndrome research?
Understanding X chromosome silencing has significant implications for Rett syndrome research. Since the disorder is caused by mutations on the X chromosome, insights into the inactivation process can help devise strategies to activate the silenced healthy gene, offering potential therapeutic avenues. Jeannie Lee’s lab is exploring how to manipulate these mechanisms to develop treatments aimed specifically at ameliorating the symptoms of Rett syndrome.
What discoveries have been made regarding the ‘Jell-O’ substance related to X chromosome inactivation?
Recent discoveries by Jeannie Lee’s lab highlight the importance of a gelatinous substance, referred to as ‘Jell-O,’ which separates chromosomes and plays a pivotal role in X chromosome inactivation. This substance is altered by Xist RNA, allowing for effective chromosomal silencing. These findings not only enhance our understanding of chromosomal organization but also provide a potential target for therapeutic strategies aimed at tackling genetic disorders linked to mutations on the X chromosome.
How do researchers study X chromosome inactivation to improve treatments for genetic disorders?
Researchers study X chromosome inactivation by examining the molecular interactions that occur during the process, particularly focusing on how Xist RNA interacts with the surrounding chromosomal material. By manipulating these processes in laboratory settings, as seen in Jeannie Lee’s lab, scientists aim to develop treatments that can restore gene function in individuals affected by genetic disorders such as Fragile X and Rett syndromes, ultimately leading to clinical applications in disease management.
| Key Point | Description |
|---|---|
| X Chromosome Inactivation | Females have two X chromosomes, but only one functions, requiring inactivation of the other. |
| Role of Xist RNA | Xist RNA modifies the surrounding ‘Jell-O’ substance, facilitating the inactivation process. |
| Discovery by Jeannie T. Lee | Lee’s lab has made significant advances in understanding how X-inactivation occurs at a molecular level. |
| Potential Therapies | Research suggests that understanding X-inactivation could lead to treatments for Fragile X and Rett syndromes. |
| Future Research | Continued studies will focus on optimizing treatments and moving towards clinical trials to help affected individuals. |
Summary
X chromosome inactivation is a crucial biological process that allows females to manage their two copies of the X chromosome efficiently. This intricate mechanism, uncovered through decades of research led by Jeannie T. Lee and her colleagues, not only provides insight into fundamental cellular operations but also opens the door to potential therapies for genetic disorders linked to mutations on the X chromosome. As ongoing research seeks to unravel remaining mysteries and perfect therapeutic approaches, the hope is that this groundbreaking work will lead to effective treatments for conditions like Fragile X Syndrome and Rett Syndrome.